Delphi Methodology 
A 2-stage modified Delphi consensus process was conducted as part of Aim 1 of this project, which includes creation of a minimum set of outcomes and associated measurement instruments for use in all clinical research studies that are planning to evaluate acute respiratory failure/acute respiratory distress syndrome (ARF/ARDS) survivors after hospital discharge.
The protocol developed for our modified Delphi process can be found under Aim 1 on the “About us” page.
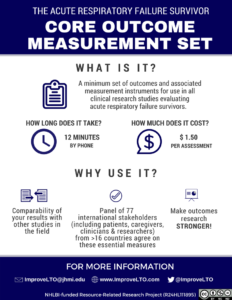
Core Outcomes Measures for Patient-Centered Clinical Research in Acute Respiratory Failure Survivors
Resources:
Generic templates and resources to aid researchers interested in implementing a modified Delphi consensus process.
Templates:
- Contact Information Form
- Communication Templates (E-mail templates)
- First Reminder to Stakeholder Organization for Representative
- Second Reminder to Stakeholder Organization for Representative
- Invitation to Representative of Stakeholder Organization
- Acknowledgement to Representative of Stakeholder Organization
- Registration Stage 1 Round 1
- Registration Stage 1 Round 1 Reminder
- Stage 1 Round 2
- Stage 1 Round 2 Reminder
- Post-Delphi Survey for panel members | Post Delphi Survey results are here
- Instrument Card
References:
- Akinremi A, Turnbull AE, Chessare CM, Bingham III CO, Needham, Dinglas VD. Delphi panelists for a core outcome set project suggested both new and existing dissemination strategies that were feasibly implemented by a research infrastructure project. Journal of Clinical Epidemiology. 2019; 114:104-107. PubMed
- Dinglas VD, Cherukuri SPS, Needham DM. Core outcomes sets for studies evaluating critical illness and patient recovery. Curr Opin Crit Care. 2020. 26:489-499. Free Full Text
- Dinglas VD, Faraone LN, Needham DM. Understanding patient-important outcomes after critical illness: a synthesis of recent qualitative, empirical and consensus-related studies. Curr Opin Crit Care. 2018. 24:401-409. Free Full Text
- Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham III CO, Turnbull AE. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196:1122-1130. PubMed
- Turnbull AE, Sepulveda KA, Dinglas VD, Bingham CO, Needham, DM. Core domains for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Critical Care Medicine 2017; 45(6):1001-1010. PubMed
- Turnbull AE, Dinglas VD, Friedman LA, Chessare CM, Sepulveda KA, Bingham CO, Needham DM. A Survey of Delphi Panelists after Core Outcome Set Development Revealed Positive Feedback and Methods to Facilitate Panel Member Participation. Journal of Clinical Epidemiology. 2018;102:99-106. Article
Helpful links:
Core Outcome Set – Methodology
- COMET (Core Outcome Measures in Effectiveness Trials) Initiative
- Standardising outcomes for clinical trials and systematic reviews
- COMET DelphiManager Online Software
- Students for Best Evidence – Delphi Consensus Technique
- The COMET Handbook: version 1.0 | Video (Guidelines for developing and implementing a core outcome set)
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations (Guidance on the minimum standards for developing a COS)
- Core Outcome Set-STAndardised Protocol Items (COS-STAP Statement)
- Impact of Design Characteristics on Response Rates in Delphi Surveys within COS Development
- Choosing Important Health Outcomes for Comparative Effectiveness Research
- Developing core outcome sets for clinical trials: issues to consider.
- A RCT on the impact of question order on prioritization of outcomes in the development of a core outcome set
- Methodological analysis of patient involvement the development of Core Outcome Set
- Delphi procedure in COS development: Rating scale and consensus criteria determined outcome selection
- OMERACT COS Methodology: A Brief Video
- OMERACT COMS Methodology: A Brief Video
- Rating Scale & Consensus Criteria Associated with Consensus Outcomes
- Sharing of results after each Delphi round influences voting in next round
- Minimum sample size of multistakeholder Delphi surveys to stabilize replicability of results
Reporting and Dissemination
- Core Outcome Set-STAndards for Reporting: The COS-STAR Statement (Guidance on the minimum standards for reporting a COS)
- Ideas to optimize dissemination of core outcome sets
- The COMET Handbook: version 1.0 (Guidelines for developing and implementing a core outcome set)
- Center for Medical Technology Policy (CMTP) COS Resources
Other
- Operations Manual for abstracting data for a Scoping Review of ICU Post-discharge Outcomes
- PROMIS: Patient-Reported Outcomes Measurement Information System
- Background on project for improving uptake of Core Outcome Sets (COS)
- Recruitment and Retention of Participants in e-Delphi surveys: the COMiT’ID study
- Checklist for Selecting Outcome Measures
- COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN)