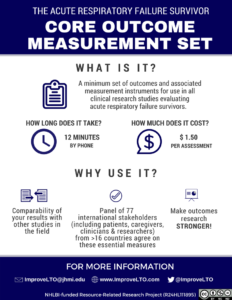
Aim 1 of this project includes creation of a minimum set of outcomes and associated measurement instruments for use in all clinical research studies that are planning to evaluate acute respiratory failure/acute respiratory distress syndrome (ARF/ARDS) survivors after hospital discharge.
A 2-stage modified Delphi consensus process was conducted with representatives from relevant stakeholder groups, including:
(1) clinical researchers from >16 countries from 6 continents,
(2) clinicians from US, Canada, UK, and Australia,
(3) ICU survivors/caregivers from US, Canada, UK, and Australia, and
(4) representatives of US federal funding bodies.
For more information about the process, please see: Modified Delphi Consensus Protocol. Visit the Core Outcome Set Resources page to locate generic templates and resources to aid researchers interested in implementing a Delphi consensus process.

A NIH National Heart, Lung, and Blood Institute working group recommended this core outcome measurement set (COMS) for all studies evaluating post-hospital patient outcomes. (PubMed) Furthermore, a 2019 Society of Critical Care Medicine consensus conference on evaluating post-intensive care syndrome (PICS) in adult ICU survivors recommended the outcome measures from this project’s COMS. (PubMed)
An American Thoracic Society/European Respiratory Society task force recommended this project’s COMS as part of post-discharge follow-up of acute respiratory failure survivors with COVID-19. (PubMed)
A consensus meeting (SCEPTER III group) recommended that ICU sedation trials planning to evaluate post-hospital outcomes include this project’s COMS. (PubMed)

The first stage of the Delphi consensus process established recommendations for a Core Outcome Set (COS).1 Core outcomes are defined as patient outcomes, health-related conditions, or aspects of health, that are essential to evaluate in all studies within a specific clinical field.
The following 8 core outcomes are recommended for evaluation:
- Survival
- Satisfaction with Life and Personal Enjoyment (Health-related quality of life)
- Mental health
- Pain
- Cognition*
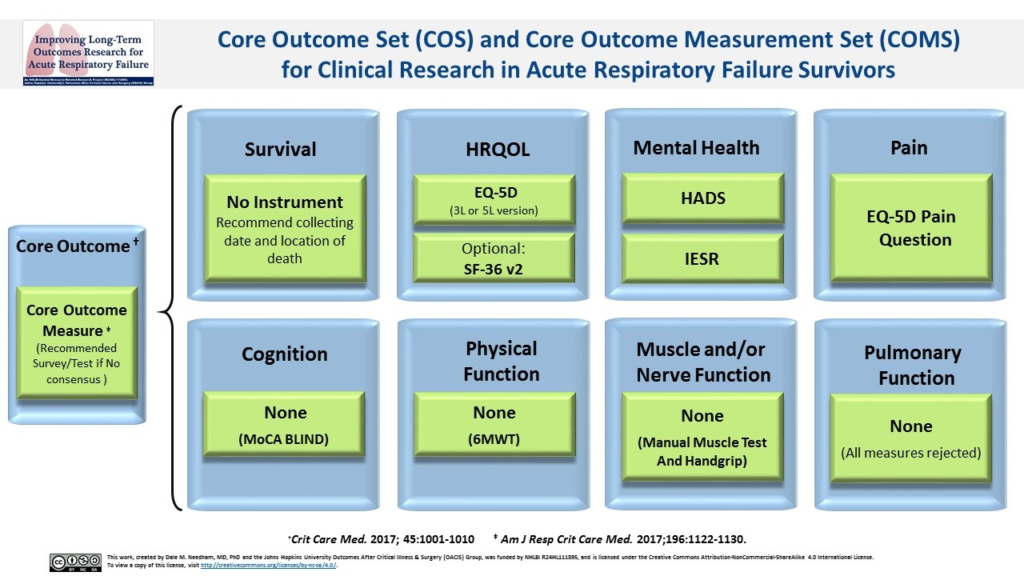
(Click to Enlarge) - Physical function**
- Muscle and/or nerve function**
- Pulmonary function**
Click here: Downloadable infographic summary of COS and COMS
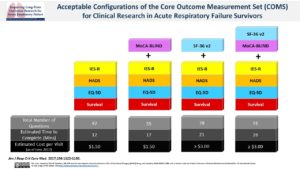
The second stage of the Delphi process established a Core Outcome Measurement Set of recommended measurement instruments for the Core Outcomes Set.2 We summarized acceptable configurations of the Core Outcome Measurement Set, along with information about total number of questions, estimated time to complete, and estimated costs per visit.
- Click here: Downloadable summary of the COMS
- Click here: Downloadable infographic of acceptable COMS configurations
Click here: Example publications & study protocols reporting an acceptable configuration of the COMS
Summary Table of Core Outcome Measurement Set Configurations (Click arrow to view) *Although there was no consensus on a cognition measurement instrument, the Montreal Cognitive Assessment (MoCA) received the highest rating and has a version (i.e., MoCA-BLIND) that can be done via phone, like the rest of the Core Outcome Measurement Set; therefore, we encourage inclusion of this instrument in the Core Outcome Measurement Set. To access the MoCA instruments, visit www.mocatest.org. ** There was no consensus on measurement instruments to include for these outcomes. However, if in-person assessments are part of a research protocol, the following tests received the highest scores from the Delphi panel: Physical Function (6-Minute Walk Test); Muscle and/or Nerve Function (Manual Muscle Test and Hand-Grip Strength); and Pulmonary Function (panel voted to exclude all tests that were considered (e.g., spirometry, St. George’s Respiratory Questionnaire)). *** To access IES-R, see Table 1 in Critical Care. 2019:23:362. Link to Article.Core Measure Set Name Measurement Instruments Total No. of Questions Estimated Time to Complete (minutes) Estimated Cost per Visit
(as of June 2017)Minimum Set Survival measure, EQ-5D, HADS, IES-R 42 12 minutes $1.50 Minimum + cognitive screening Survival measure, EQ-5D, HADS, IES-R, + MoCA 55 17 minutes $1.50 Minimum + SF-36 Survival measure, EQ-5D, HADS, IES-R, + SF-36 v2 78 21 minutes ≥$3.00 Minimum + SF-36 + cognitive screening Survival measure, EQ-5D, HADS, IES-R, + SF-36 v2 + MoCA 91 26 minutes ≥$3.00
For more information about each measure, please log-in to the website to access more FREE information about the Core Outcome Measure Set. (Login / Register) </br>
Core Outcome Measurement Set is available in ≥15 Languages
| Language | EQ-5D | HADS | IES-R | SF-36 V2 | MoCA-BLIND |
|---|---|---|---|---|---|
| English | ✓ | ✓ | ✓ | ✓ | ✓ |
| Spanish | ✓ | ✓ | ✓ | ✓ | ✓ |
| French | ✓ | ✓ | ✓ | ✓ | ✓ |
| German | ✓ | ✓ | ✓ | ✓ | ✓ |
| Japanese | ✓ | ✓ | ✓ | ✓ | ✓ |
| Chinese | ✓ | ✓ | ✓ | ✓ | ✓ |
| Dutch | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hebrew | ✓ | ✓ | ✓ | ✓ | ✓ |
| Korean | ✓ | ✓ | ✓ | ✓ | ✓ |
| Norwegian | ✓ | ✓ | ✓ | ✓ | ✓ |
| Russian | ✓ | ✓ | ✓ | ✓ | ✓ |
| Swedish | ✓ | ✓ | ✓ | ✓ | ✓ |
| Turkish | ✓ | ✓ | ✓ | ✓ | ✓ |
| Greek | ✓ | ✓ | ✓ | ✓ | ✓ |
| Lithuanian | ✓ | ✓ | ✓ | ✓ | ✓ |
| Farsi | ✓ | ✓ | ✓ |
American Thoracic Society Journal Club Recording – Core Outcome Measures
References 2. Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham III CO, Turnbull AE. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196:1122-1130. PubMed Helpful links NOTE: If you implement this Core Outcome Measurement Set, we request that you also obtain feedback from participants and research staff via a very brief 6 questions survey. Please contact [email protected] to learn more about this Core Outcome Measurement Set survey for participants and researchers. For more information or questions, contact [email protected].
