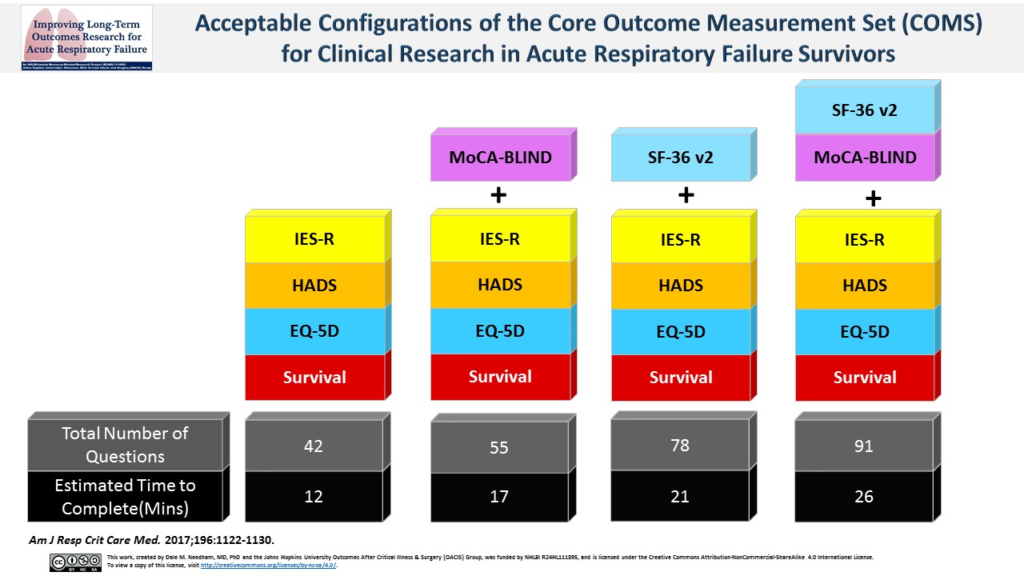
A NIH National Heart, Lung, and Blood Institute working group recommended this core outcome measurement set (COMS) for all studies evaluating post-hospital patient outcomes. PubMed
Below are example publications and study protocols that report all components of the Core Outcome Measurement Set (COMS) for post-hospital research of acute respiratory failure survivors.
To have publications and study protocols added to this list, please email us at [email protected] or via our contact us page.
Publications:
Minimum COMS
- Azoulay E, Resche-Rigon M,…, Kentish-Barnes N. One-year outcomes in acute respiratory distress syndrome survivors: COVID-19 versus seasonal influenza or pneumonia. Intensive Care Med. 2024 May;50(5):773-775. Article | PubMed (SF-36 instead of EQ-5D)
- Cox CE, Hough CL,…,Porter LS. Effects of a telephone- and web-based coping skills training program compared to an education program for survivors of critical illness and their family members: a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine. 2018; 197(1):66-78. PubMed
- Derry HM, Lief L, Woubeshet N, Schenck EJ, Kakarala S, LaFond E, Berlin DA, Prigerson HG. Peritraumatic Stress Symptoms during Early Post-ICU Recovery. Ann Am Thorac Soc. Sep 1 2020. PubMed
- Demoule A, Hajage D,…, Similowski T. Prevalence, intensity and clinical impact of dyspnea in critically ill patients receiving invasive ventilation. Am J Respir Crit Care Med. 2022 Jan 21. In Press PubMed (SF-36 instead of EQ-5D)
- Geense WW, Zegers M, Peters MAA, Ewalds E, Simons KS, Vermeulen H, van der Hoeven JG, van den Boogaard M. New Physical, Mental, and Cognitive Problems 1-year Post-ICU: A Prospective Multicenter Study. Am J Respir Crit Care Med. 2021 Feb 1. PubMed (SF-36 instead of EQ-5D)
- Geense WW, Zegers MAA, Vermeulen H, van den Boogaard M, van der Hoeven J. MONITOR-IC study, a mixed methods prospective multicenter controlled cohort study assessing 5-year outcomes of ICU survivors and related healthcare cost: a study protocol. BMJ Open. 2017 Nov 14;7(11):e018006. doi: 10.1136/bmjopen-2017-018006. PMID: 29138206; PMCID: PMC5695418. PubMed (SF-36 instead of EQ-5D)
- Heesakkers H, Hoeven JG,…, Boogaard M. Mental health symptoms in family members of COVID-19 ICU survivors 3 and 12 months after ICU admission: a multicentre prospective cohort study. Intensive Care Medicine. 2022 Feb 1. Article PubMed (SF-12 instead of EQ-5D)
- Hellemons ME, Huijts S,…,Aerts JGJV. Persistent health problems beyond pulmonary recovery up to 6 months after hospitalization for COVID-19: a longitudinal study of respiratory, physical, and psychological outcomes. Annals of the American Thoracic Society. 2022 Apr;19(4):551-561. Article | PubMed (SF-36 instead of EQ-5D)
- Hodgson CL, Udy AA,…, Cooper DJ. The impact of disability in survivors of critical illness. Intensive Care Med. 2017. PubMed
- Liu K, Nakashima T,…, Ogura T; ILOSS Study Group. Phenotypes of Functional Decline or Recovery in Sepsis ICU Survivors: Insights From a 1-Year Follow-Up Multicenter Cohort Analysis. Crit Care Med. 2025 Feb 24. Epub ahead of print. Article |PubMed
- McGregor G, Sandhu H, …, Underwood M. Clinical effectiveness of an online supervised group physical and mental health rehabilitation programme for adults with post-covid-19 condition (REGAIN study): multicentre randomised controlled trial. BMJ. 2024 Feb 7;384:e076506. Article | PubMed
- McGregor G, Sandhu H,…, Underwood M. Rehabilitation Exercise and psycholoGical support After COVID-19 InfectioN (REGAIN): a structured summary of a study protocol for a randomized controlled trial. Trials. 2021; 22:8. Article PubMed
- Tripathy S, Acharya SP,…, Kar N. Post traumatic stress symptoms, anxiety, and depression in patients after intensive care unit discharge-a longitudinal cohort study from a LMIC tertiary care centre. BMC Psychiatry. 2020; 20(1):220. Article PubMed
- Tripathy S, Kar N,…, Singh SK. ICU memories and patient outcomes in a low middle-income country: a longitudinal cohort study. Crit Care Med. 2021; 49(10):e978-e988. Article PubMed
- Unoki T, Sakuramoto H, Uemura S,…, Masuda Y. Prevalence of and risk factors for post-intensive care syndrome: Multicenter study of patients living at home after treatment in 12 Japanese intensive care units, SMAP-HoPe study. 2021.16(5):e0252167. doi: 10.1371/journal.pone.0252167. Article; PubMed (in Japanese)
- van der Heijden EFM, Kooken RWJ, …, van den Boogaard M. Differences in long-term outcomes between ICU patients with persistent delirium, non-persistent delirium and no delirium: A longitudinal cohort study. J Crit Care. 2023 Feb 17. doi: 10.1016/j.jcrc.2023.154277. PubMed |
- van Gelder TG, Lalmohamed A, …, Slooter AJC. Systemic glucocorticoid use during ICU admission and symptoms of posttraumatic stress disorder in intensive care unit survivors. Intensive Care Med. 2022 Jun;48(6):762-764. doi: 10.1007/s00134-022-06725-x. PubMed |
- Wang S, Xin H, Chung C,…, Hu RF. Effect of an ICU diary on psychiatric disorders, quality of life, and sleep quality among adult cardiac surgical ICU survivors: a randomized controlled trial. Critical Care. 2020. 24(1). 81. PubMed (SF-36 instead of EQ-5D)
- Watanabe S, Liu K,…, Kotani T. Association between early mobilization in the ICU and psychiatric symptoms after surviving a critical illness: a multi-center prospective cohort study. Journal of Clinical Medicine. 2022; 11(9):2587. doi: 10.3390/jcm11092587.
- Wu TT, Chen QL, …, Li H. Effects of a multilevel intervention of resistance training with or without beta-hydroxy-beta-methylbutyrate in medical ICU patients during entire hospitalisation: a four-arm multicentre randomised controlled trial. Crit Care. 2023 Dec 15;27(1):493. PubMed |
- Wong HZ, Brusseleers M,…, Gluck S. Mixed-mode versus paper surveys for patient-reported outcomes after critical illness: a randomised controlled trial. Australian Critical Care. 2022. 35(3):286-293. PubMed |
Minimum COMS + SF-36
- Bels JLM, Thiessen S, …, Mesotten D; PRECISe study team. Effect of high versus standard protein provision on functional recovery in people with critical illness (PRECISe): an investigator-initiated, double-blinded, multicentre, parallel-group, randomised controlled trial in Belgium and the Netherlands. Lancet. 2024 Aug 17;404(10453):659-669. PubMed
- Berney S, Hopkins RO, Rose JW, Koopman R, Puthucheary Z, Gordon I, Colantuoni E, Parry SM, Needham DM, Denehy L. Functional electrical stimulation in-bed cycle ergometry in mechanically ventilated patients: a multicenter randomized controlled trial. Thorax. 2020; Dec 15;
- Dinglas VD, Hopkins RO,…, Needham DM. One-year outcomes of rosuvastatin vs. placebo in sepsis-associated acute respiratory distress syndrome: prospective follow-up of SAILS randomized trial. Thorax. 2016; 71: 401-410. PubMed
- Ishinuki T, Zhang L, …, Mizuguchi T. Clinical impact of rehabilitation and ICU diary on critically ill patients: A systematic review and meta‐analysis. Nursing in Critical Care. 2023; Jan 9. Article
- Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, Hopkins RO. One year outcomes of initial trophic vs full enteral feeding in acute lung injury patients: prospective follow-up of the EDEN randomized trial. British Medical Journal. 2013; Mar 19;346:f1532. PMCID: PMC3601941
- Ruhl AP, Huang M,…, Needham DM. Health care resource use and costs in long-term survivors of ARDS: a 5-year longitudinal cohort study. Critical Care Medicine. 2017;45:196-204. PubMed
- Tramm R, Ilic D, Sheldrake J, Pellegrino V, Hodgson C. Recovery, risks, and adverse health outcomes in year 1 after extracorporeal membrane oxygenation. American Journal of Critical Care. 2017;26(4):311-319. PubMed
- Vlake JH, Van Bommel J,…,Michel E. Psychologic distress and quality of life after ICU treatment for coronavirus disease 2019: a multicenter, observational cohort study. Critical Care Explorations. 2021; 3(8): e0497. PubMed
Minimum COMS + MoCA
- Brown SM, Dinglas VD, …, Needham DM; APICS-01 Study Team. Association between unmet medication needs after hospital discharge and readmission or death among acute respiratory failure survivors: the addressing post-intensive care syndrome (APICS-01) multicenter prospective cohort study. Crit Care. 2022 Jan 7;26(1):6. PubMed |
- Castro-Avila A, Merino-Osorio C, G…, Leppe J; on behalf of the IMPACCT COVID-19 study group. Six-month post-intensive care outcomes during high and low bed occupancy due to the COVID-19 pandemic: A multicenter prospective cohort study. PLoS One. 2023 Nov 16;18(11):e0294631. PubMed |
- Hodgson, C.L., Higgins, A.M…, The PREDICT Study Investigators. Comparison of 6-month outcomes of sepsis versus non-sepsis critically ill patients receiving mechanical ventilation. Critical Care. 2022 Jun 13;26(1):174. PubMed
- Maley JH, Sandsmark DK,…,Lane-Fall MB. Six-month impairment in cognition, mental health, and physical function following COVID-19-associated respiratory failure. Critical Care Explorations. 2022 Mar 28;4(4):e0673. PubMed |
- Rousseau AF, Minguet P,…,Misset B. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Intensive Care. 2021. 11:118. PubMed
- TEAM Study Investigators and the ANZICS Clinical Trials Group, Hodgson CL, …, Young PJ. Early Active Mobilization during Mechanical Ventilation in the ICU. N Engl J Med. 2022 Nov 10; 387(19):1747-1758. PubMed |
Minimum COMS + MoCA + SF-36
- Berentschot, J.C., Bek, L.M., …, Hellemons M. E., van den Berg-Emons, H. J. G. on behalf of The CO-FLOW collaboration Group. Long-term health outcomes of COVID-19 in ICU- and non-ICU-treated patients up to 2 years after hospitalization: a longitudinal cohort study (CO-FLOW). Journal of Intensive care 12, 47 (2024). Article
Study Protocols:
- A2B Trial – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- APICS – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- APICS-COVID – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- BEHAB – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- COVID-Recovery – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- GRAIL – detailed information found at ClinicalTrials.gov (Minimum COMS)
- icuRESOLVE – detailed information found at ANZCTR.org.au (Minimum COMS)
- IMPACCT COVID-19-detailed information found at ClinicalTrials.gov (Minumum COMS +MoCA-Blind)
- MiCare Trial – detailed information found at ClinicalTrials.gov | PMCID: PMC9478839 | (Minimum COMS)
- NEXIS Study – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- PRAISE Study – detailed information found at ClinicalTrials.gov | (Minimum COMS)
- PRECISe Trial – detailed information found at ClinicalTrials.gov (Minimum COMS + SF36)
- PREDICT Study – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- PSBPS Study – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- TEAM III – detailed information found at ClinicalTrials.gov (Minimum COMS + MoCA-Blind)
- Quality of Life and Patient-centered Outcomes After Hospitalization for COVID-19 – Detailed information found at ClinicalTrials.gov (Minimum COMS)